Review of Technologies for Biopharmaceutical Higher Order Structural (HOS) Analysis
Scientists and regulators have become increasingly aware of the critical role that protein HOS plays in ensuring a drug’s stability, safety, and biological function. The FDA, along with the Center for Drug Evaluation and the Center for Research Biologics Evaluation and Research, issued recommendations that drug companies employ state-of-the art technology for evaluating HOS.
Although a variety of traditional HOS analysis techniques and technologies are available, these approaches have shown deficiencies in reliably predicting efficacy and safety, establishing the need for new and improved HOS analyses.
Let us review the available HOS analysis options to determine the most robust, cost-effective, and workflow-friendly approach for your lab.
HOS Analysis can be Grouped into Low-, Mid-,
and High-Resolution Techniques
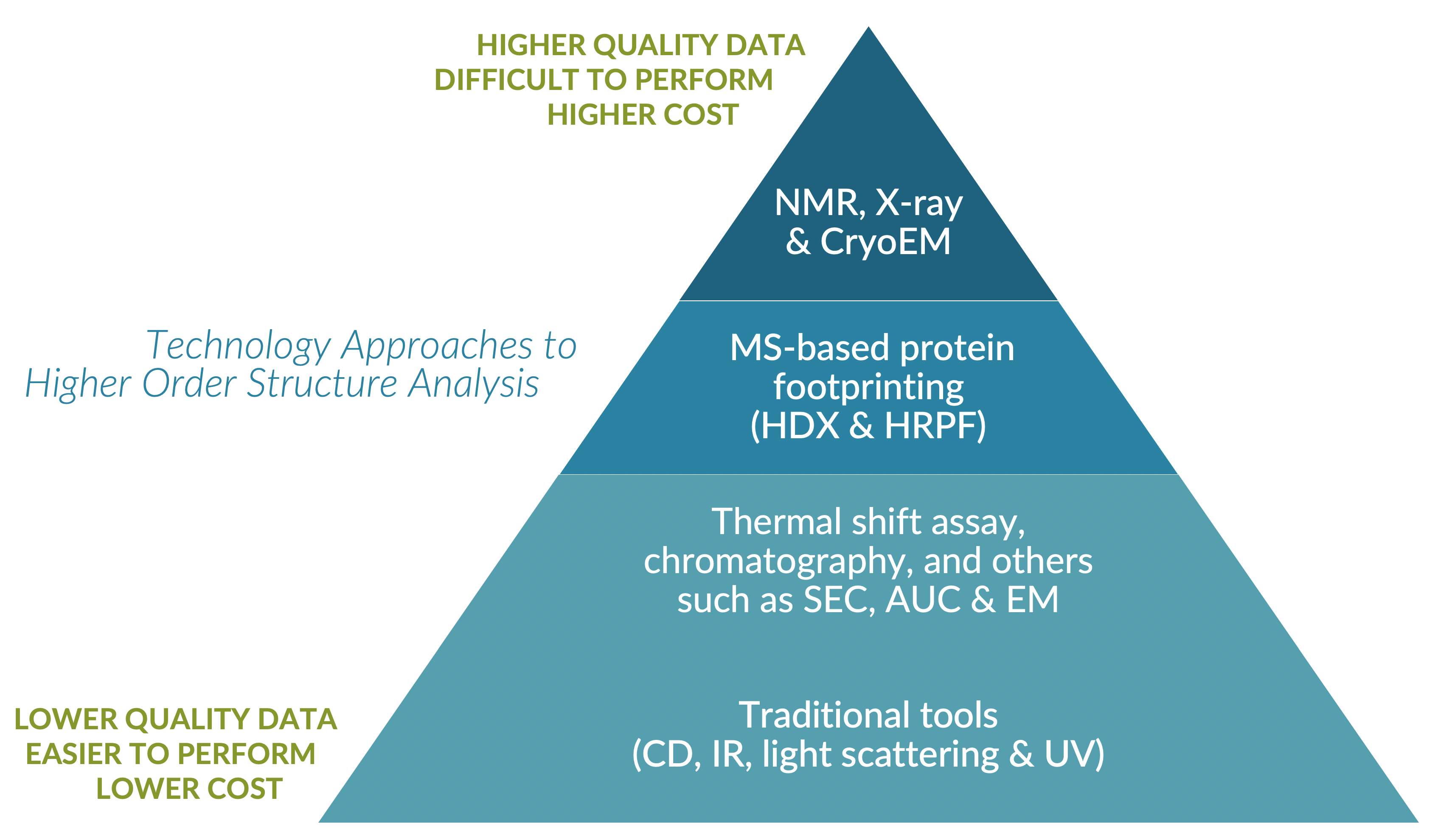
Low-Resolution Methods
Low-resolution methods provide information that is spatially averaged over the entire protein population. These methods examine a very limited number of specific moieties in the protein structure that have altered biophysical properties dependent upon protein conformation.
While more widely available and easiest to use, low-resolution techniques fail to inform on a residue-level, and as such, yield ambiguous and marginally actionable HOS data.
High-Resolution Methods
High-resolution techniques such as x-ray crystallography, multi-dimensional nuclear magnetic resonance (NMR), and cryo-electron microscopy (CryoEM), demand deep expertise and very expensive equipment. Beyond these complexities, each also has idiosyncratic technical challenges that limit their value for HOS studies as follows.
X-Ray Crystallography
The adoption of x-ray crystallography in HOS studies has been limited by the scarcity of electron beam sources, which is aggravated by difficulties in crystallizing large bio-therapeutics such as monoclonal antibodies.
Multi-Dimensional Nuclear Magnetic Resonance
NMR is arguably the most sensitive method for fingerprinting the HOS of a protein in solution. Yet, two-dimensional NMR can yield poor selectivity which is problematic for unambiguous fingerprinting of biological macromolecules. And this method is quite laborious and costly, consuming considerable time and sample to properly assign multi-dimensional spectra.
Cryo-Electron Microscopy
The utility of Cryo-EM is predominantly limited to the study of large proteins (>100 kDa) and demands substantial sample preparation and purification to ensure the absence of impurities.
Mid-Resolution Methods
Intermediate resolution techniques include mass spectrometry (MS) combined with protein footprinting using surface chemical modification and/or amide Hydrogen-Deuterium Exchange (HDX).
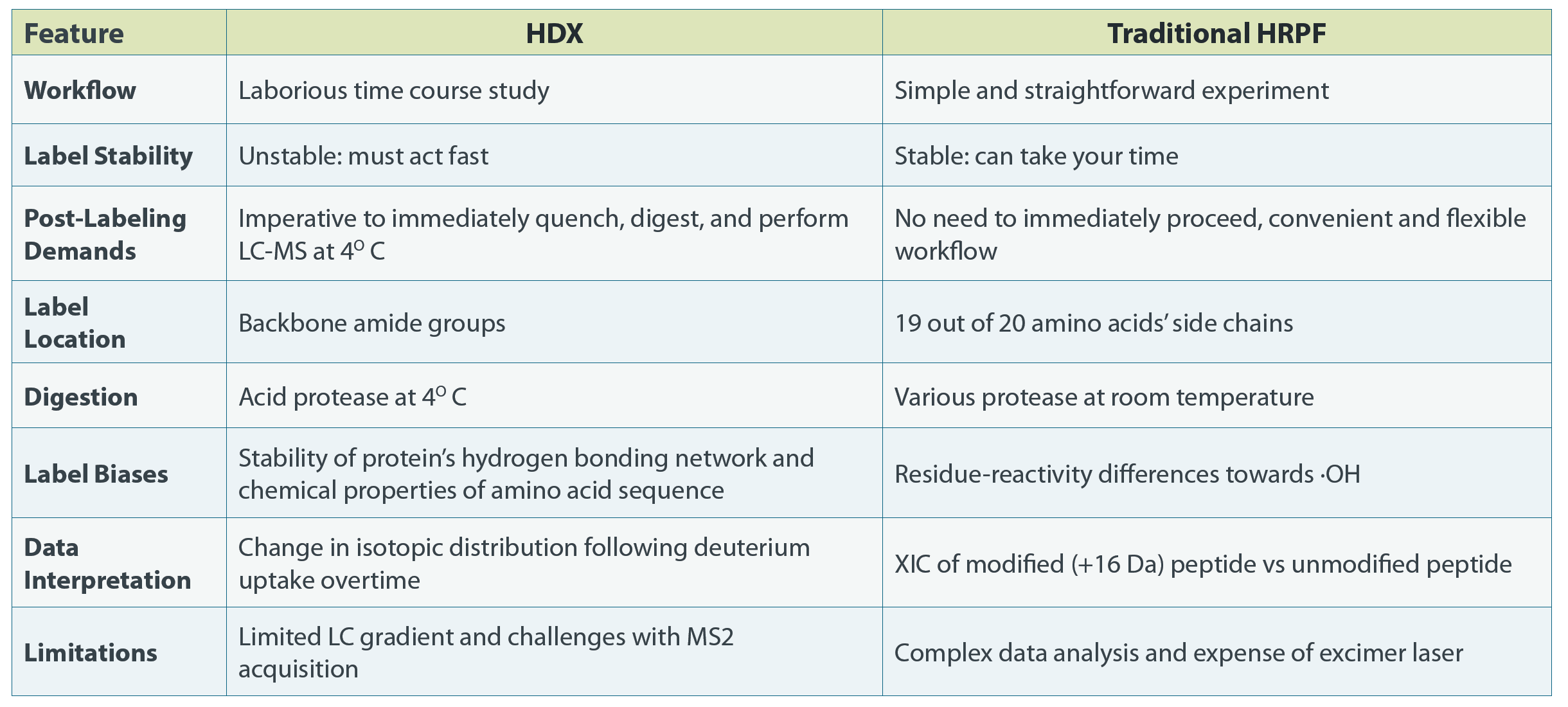
Hydrogen-Deuterium Exchange
Because integrated commercial packages for data acquisition and analysis are available, HDX-MS has been widely used in biopharmaceutical research.
In HDX studies, electrospray ionization MS detects mass increases from exchanged hydrogen with deuterium. The back-exchange of deuterium to hydrogen occurs at substantial rates, requiring the reaction to be quenched rapidly by cooling the sample to 1°C while suspended in acidic buffer (pH 2.7). The quenching must be promptly followed by peptic digestion and then LC-MS analysis.
While HDX can provide insight into solvent addressable backbone amide hydrogens, its time-course incubation often results in generating products from both rapid and slow exchange processes. Rapid exchange is associated with solvent addressable residues with unbound amide hydrogens, while slower reactions occur with internal, titratable, backbone amide protons involved in hydrogen bonds that make up secondary structural elements, whose exchange rates are driven by conformational change. The net result is an ambiguous understanding of HOS and secondary structure stability.
Performing HDX HOS analysis is a complex and burdensome process. Perhaps this point is best exemplified by Professor Mark Chance of Case Western Reserve University when he remarked, “I invented HRPF because HDX is just too hard to do.”
Hydroxyl Radical Protein Footprinting (HRPF)
HRPF is an intermediate level technique that utilizes hydroxyl radicals (•OH) to covalently modify solvent-exposed amino acid side chains. The •OH modification is very stable, allowing ample time to denature and digest the protein for bottom-up proteomics after labeling.
The average peptide and residue oxidation is calculated using the chromatographic peak areas of the unmodified and modified peptides. When solvent accessibility changes due to alterations in protein structure or interactions, •OH modification concordantly changes.
Researchers have used HRPF to successfully:
- detect defects in protein HOS and function,
- expose monoclonal antibody (mAb) production problems,
- demonstrate the interplay of mAb HOS and drug function,
- illustrate biosimilar failure and storage-induced defects for innovator products,
- characterize membrane protein targets,
- determine protein conformational change and binding Kd upon ligand complexation,
- facilitate the study of allostery, and
- enable in vivo labeling of cellular proteins and whole organisms.
Factors Impeding the Adoption of Traditional HRPF in HOS Studies
While the attributes of traditional HRPF are impressive, its adoption within pharma has been slow because in the past the only means to quickly generate •OH required scarcely-available, elaborate x-ray synchrotron beamlines or expensive, dangerous, and hard to maintain excimer lasers using hazardous KrF gas.
Additionally, •OH can react with non-analyte components in the sample, such as buffer and excipients. Variability in the rate of background scavenging is associated with irreproducibility, which has limited comparative studies.
Overall, older HRPF approaches have shown obvious advantages over low-resolution techniques, while generating results compatible with high-resolution approaches. However, the battles using these HRPF methods are hard-won. With the introduction of the world’s first commercially available HRPF instrument and software platform, GenNext Technologies has changed all that.
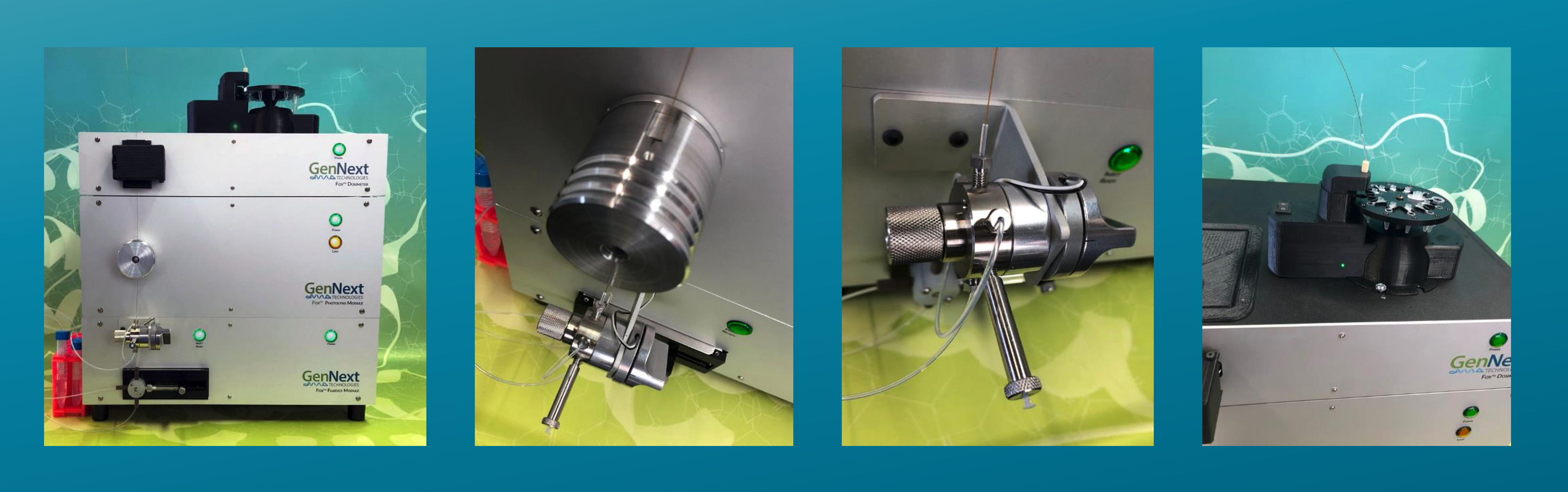
Flash Oxidation (Fox®) Protein Footprinting System
The Fox System is the world’s only benchtop platform available for Hydroxyl Radical Protein Footprinting studies
In Fox-based Hydroxyl Radical Protein Footprinting (HRPF), protein is pre-mixed with H202 and adenine and is introduced into the photolysis module by pumping sample through a fused-silica capillary. Adenine serves as an internal standard whose change in photometric absorbance reflects effective •OH yield.
At the photolysis module, photo-irradiation generates •OH that attack the protein and adenine. The sample continues to flow upwards and into the detection zone of the radical dosimeter, where the change in UV absorbance is measured. During labeling, the dosimetry module assesses the effective •OH load, enabling the user to adjust flash intensity in response to changes in background scavenging. This unique, real-time feedback loop vastly improves labeling reproducibility, providing confidence in differential studies. At the product collector, labeled protein is ultimately deposited in microtubes containing a quench solution of catalase and methionine.
The Fox Protein Footprinting System can easily perform all the HRPF-based HOS studies that previously required complicated, expensive, and dangerous laser or beamline sources.