On-Demand Presentations by the GenNext Team on the Flash Oxidation (Fox®) Protein Footprinting System
Presented by Dr. Joshua Sharp from The University of Mississippi
This is an expanded version of the talk Dr. Joshua Sharp gave at the American Society for Mass Spectrometry Annual Conference in 2024. In it, he covers the initial development of radical protein footprinting in mammalian whole blood, a new technology for clinical structural proteomics.
Watch>
Presented by Dr. Zhi Cheng, Applied Research Scientist at GenNext Technologies, Inc.
Enhance your understanding of protein High Order Structure (HOS) for therapeutics with protein footprinting technology coupled to high-resolution mass spectrometry using both CID and EAD peptide fragmentation.
The HOS of protein therapeutics is integral to a drug’s stability, safety, and biological function. Incorrect configurations of HOS or interactions with therapeutic targets can lead to adverse drug reactions. Hydroxyl Radical Protein Footprinting (HRPF), utilizing the Fox® Protein Footprinting System, enables the exploration of the effects of epitope/paratope mapping, aggregation-interface identification, formulation, small molecule binding, and other factors, thereby enhancing the understanding and significance of therapeutic HOS.
Watch>
Presented by Dr. Michael Gross, Washington University in St. Louis
FPOP, first demonstrated in 2005, has proven to be a versatile tool for problem solving in protein chemistry and physics. Given the microsecond speed of the footprinting, FPOP can follow fast folding/unfolding of protein and map the regions of the protein that are undergoing the conformational change. Its speed also permits location of regions with hidden conformational changes. In biopharmaceutical applications, it can identify protein conformational changes owing to thermal stress and can be productive in epitope mapping. It can also map conformational changes occurring upon binding to small ligands and metal ions, allowing metal-ion affinity and order of binding to be determined. A particularly relevant application in understanding Alzheimer’s and other neurodegenerative diseases is mapping amyloid formation especially of soluble oligomers, the purported toxic substances in the disease. A current application where few biochemical tools are applicable is the emerging field of membrane proteins. Footprinting, both in membrane mimetics (e.g., nanodisks and liposomes) and in living cells, may be a significant option. The lecture will describe FPOP and cover applications and make comparisons with hydrogen/deuterium exchange and footprinting with slow chemical reactions occurring on the minutes time scale.
Watch>
Presented by Emily Chea, GenNext Technologies
Utilizing the Fox System, researchers have explored the effects of epitope/paratope mapping, aggregation-interface identification, formulation, small molecule binding, and other factors, thereby enhancing the understanding and significance of therapeutic HOS. The Fox System is a novel HRPF technique that utilizes hydroxyl radicals (•OH) to irreversibly modify solvent-exposed amino acid side chains. As amino acids’ solvent accessibility changes, the •OH modification concordantly changes. This uncovers regions buried or exposed after changing conditions, down to the amino acid resolution. This presentation will provide background on the Fox System, how it is being applied to characterize biotherapeutic HOS, and future advancements to the Fox System innovation.
Watch>

Presented by Emily Chea, PhD
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.
WATCH >

Presented by Emily Chea, PhD
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.
WATCH >
Hacking Structural Biology for Drug Discovery Using Mass Spectrometry
Professor Mark Chance, Case Western Reserve University
Broadening the Scope of Hydroxyl Radical Protein Footprinting Applications
Professor Joshua Sharp, University of Michigan
Moving to in-cell and in-vivo Applications
Professor Lisa Jones, University of San Diego
Presented by Joshua Sharp, PhD
Learn about fundamentals of the HRPF along with applications relevant to the pharmaceutical sciences. Understand the foundations of hydroxyl radical protein labeling chemistry and the relationship of labeling to protein topography. Fox technology, the methods used to generate radicals, the method for real-time dosimetry, and the requirements for sample analysis by LC-MS/MS will be presented. Dr. Sharp will present examples of biopharmaceutical applications, while showing data highlighting higher order structure effects of formulations. Dr. Sharp will discuss the effects of different buffers on the aggregation propensity and aggregation interfaces of a therapeutic monoclonal antibody, along with verifying the ability of polysorbate to prevent aggregation without changing the topography of the monomeric protein. He will also present results defining the epitope of a therapeutic antibody.
Dr. Joshua Sharp is an internationally recognized expert in HRPF, pioneering novel MS-based technology to study HOS focusing on the structure-function relationships of proteins and carbohydrates. With more than 21 peer-reviewed articles, Dr. Sharp has also presented numerous invited lectures around the world. Along with acting as the Chief Technology Officer of GenNext, Dr. Sharp is also Associate Professor of Pharmacology, Associate Professor of Chemistry and Biochemistry, and Director of the Glycoscience Center of Research Excellence at the University of Mississippi.
WATCH >
Presented by Joshua Sharp, PhD
Dr. Joshua Sharp, Chief Technology Officer of GenNext Technologies and Associate Professor of Pharmacology, Associate Professor of Chemistry and Biochemistry, and Director of the Glycoscience Center of Research Excellence at the University of Mississippi, presents during the ABRF 2021 Virtual Meeting on the Fox Footprinting System.
Dr. Joshua Sharp is an internationally recognized expert in HRPF, pioneering novel MS-based technology to study HOS focusing on the structure-function relationships of proteins and carbohydrates. With more than 21 peer-reviewed articles, Dr. Sharp has also presented numerous invited lectures around the world. Along with acting as the Chief Technology Officer of GenNext, Dr. Sharp is also Associate Professor of Pharmacology, Associate Professor of Chemistry and Biochemistry, and Director of the Glycoscience Center of Research Excellence at the University of Mississippi.
WATCH >
 Hydroxyl Radical Protein Footprinting (HRPF) and its Relevance in Drug Development
Hydroxyl Radical Protein Footprinting (HRPF) and its Relevance in Drug Development
In the presentation, Dr. Emily Chea discusses the importance of studying the high-order structure of proteins and its relevance in drug development and characterizing biotherapeutics.
She categorizes the different techniques based on their ease of use, information value, and cost. Lower resolution techniques are common, affordable, and high throughput but provide ambiguous information about the protein’s higher-order structure. Higher resolution techniques such as X-ray crystallography, cryo-electron microscopy, and NMR offer atomic-level information but are more difficult to operate and have limitations in terms of protein size. Mass spectrometry-based techniques like chemical cross-linking, HDX (Hydrogen-Deuterium Exchange), and HRPF (Hydroxy Radical Protein Footprinting) strike a balance between ease of use and information value. Chemical cross-linking helps analyze protein complexes and interactions but can lead to non-specific modifications. HDX examines the dynamics of the protein’s backbone in a native solution state, while HRPF uses hydroxy radicals to modify exposed amino acid side chains for fast and stable labeling. HRPF is particularly useful for detecting transient interactions and complex samples. However, both HDX and HRPF have limitations in resolution and data analysis. Despite these limitations, these techniques provide valuable structural information for protein analysis and are widely used in research and drug development.
Presented by Emily Chea, PhD
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.
WATCH >
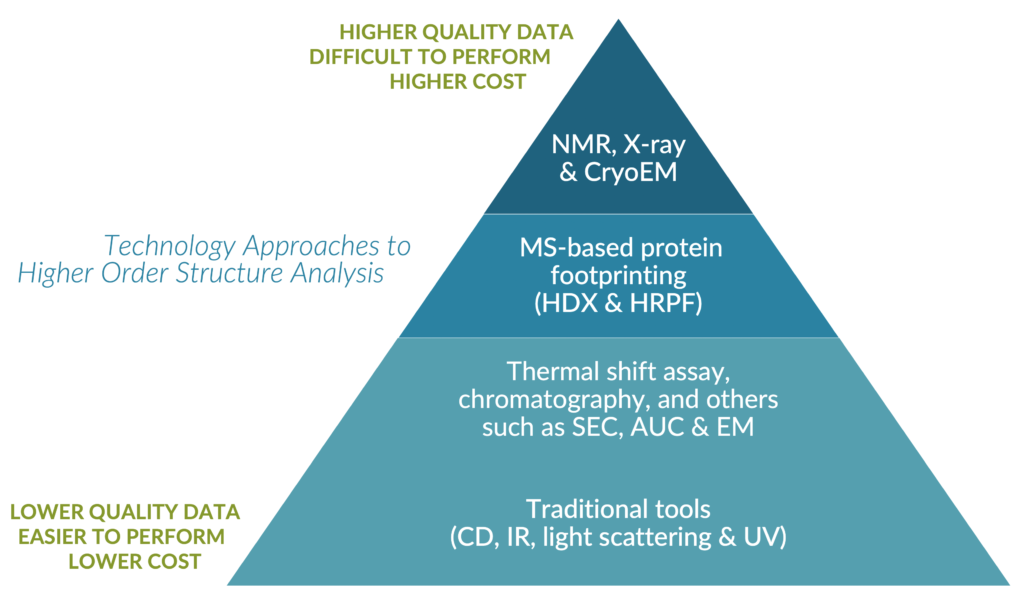
Presented by Emily Chea, PhD
Many structural biology researchers are looking for a way to make Higher Order Structural Analysis easier and more robust because they know how crucial a role it plays in biotherapuetic stability, safety, and biological function. Yet, traditional HOS approaches are known to be either complex, slow, dangerous, and expensive or unreliable, uninformative, and irreproducible. Choosing the best approach for your lab can be confusing with so many factors to consider.
Dr. Chea takes you through your options, explaining the pros and cons of each method. Then she will provide an overview of the Flash Oxidation (Fox™) Protein Footprinting System and how it is being applied in epitope mapping, mAb aggregation, and conformational analysis. By better understanding your options for higher order structure analysis, you can choose the method that empowers your lab to develop the safest and most effective biotherapeutics.
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.
WATCH >
DOWNLOAD THE TECH NOTE>
Presented by Emily Chea, PhD
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.
WATCH >
DOWNLOAD THE POSTER NOTE>

JoVE Protocol
Online method to perform flash oxidation protein footprinting using inline radical dosimetry and a plasma light sour
WATCH >
FASEB’s Protein Aggregation Conference: Function, Dysfunction, and Disease
Flash Oxidation (Fox™) Hydroxyl Radical Protein Footprinting for mAb Aggregation
Spend less than 10-minutes with Dr. Chea as she explains how the Flash Oxidation (Fox™) Protein Footprinting System can characterize monoclonal antibody (mAb) aggregation and determine how excipients help stabilize the protein. Excipients, like amino acids, can stabilize proteins and limit aggregation. By comparing the change in hydroxyl radical modification before and after the addition of an amino acid, you can detect intermolecular interactions that take place during mAb aggregation. By better understanding the higher order structure of protein aggregation, you will be armed with the information you need to optimize mAb formulation and development.
Presented by Emily Chea, PhD
Dr. Emily Chea received her PhD while studying with Professor Dr. Lisa Jones at the University of Maryland, Baltimore. Emily has made significant contributions to the development of HRPF, with particular emphasis on in-cell FPOP (IC-FPOP) for proteome-wide structural biology characterization of cellular drug–target interactions. Currently, Emily is a key member of the GenNext scientific team in her role as an Applied Research Scientist.








