
Flash Oxidation (Fox®) Protein Footprinting System
Precise control of OH radical yield with the Fox Protein Footprinting System
GenNext has obsoleted the use of expensive, complicated, and hazardous Class IV Excimer and Nd:YAG lasers with our proprietary high energy plasma photolysis source that creates impressive OH radical loads at a fraction of the cost.
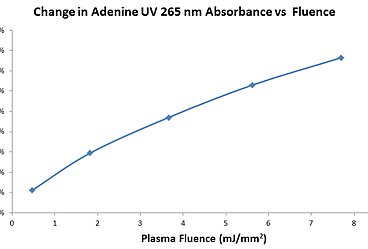
As is the case for conventional Fast Photo-Oxidation of Proteins (FPOP) for Hydroxyl Radical Protein Footprinting (HRPF), our Fox Protein Footprinting System photo-catalyzes the creation of OH radicals from H2O2, but in a highly controlled and cost-effective manner. Unlike laser systems, photolysis fluence can be precisely controlled to achieve desired OH radical yield. The interplay between photolysis fluence and OH radical yield as determined by radical dosimetry is shown in Figure 1. Fluence is regulated at precision better than +/-5 μJ per square millimeter. The photolysis pulse width is precisely controlled to less than 15 μsec, ensuring that foot-printing proceeds without the creation of unwanted HOS artifacts, typically seen in longer-term labeling approaches.
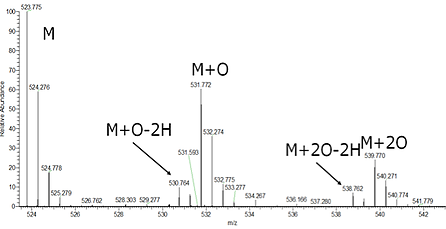
Results obtained with Fox System are eminently consistent with those of conventional FPOP, as demonstrated by the labeling and mass spectrometric analysis of Angiotensin II and protein Apo-Myoglobin. Angiotensin II was labeled with the Fox Photolysis System: 1 μM aqueous solution of Angiotensin II containing 100 mM of H2O2, 1 mM Adenine (radical dosimeter internal standard) in 100 mM phosphate buffer. The sample was irradiated using 6 mJ per square millimeter fluence. The resultant peptide profile is shown in Figure 2. As can be seen, singly oxidized and doubly oxidized products were formed, shifting the average monoisotopic mass of the labeled Angiotensin II to 529.221 m/z, consistent with results obtained by conventional FPOP (> 527 m/z).
Apo-Myoglobin was labeled in a similar fashion as Angiotensin II. As shown in Figure 3, singly, doubly, and triply oxidized products were produced. The Poisson distribution for each species indicates the absence of artifactual changes in protein HOS highly consistent with classical FPOP [15, 17, 22].
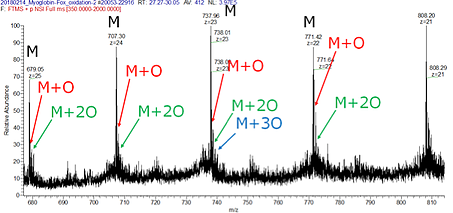
Fig. 3: Fox HRPF of Apo-Myoglobin
Contact us about integrating the Fox Photolysis System in your workflow or sign-up for a pilot project.
Fox® Radical Dosimeter
Precisely measure hydroxyl radical concentration
The Fox Radical Dosimeter is an in-line capillary UV detector located immediately downstream of the Fox Photolysis System. Real-time photometric absorbance measurements during radical production are produced, enabling the assessment of effective hydroxyl radical concentration.
While OH radicals are excellent probes of protein topography, they also react with many compounds found in biopharmaceutical preparations. Competition between biopharmaceutical and background scavengers for free OH radicals exists, making it necessary to measure the effective concentration of OH radical available to oxidize the target protein. The latter can be achieved by using an internal standard dosimeter. Ideally, an internal standard dosimeter would have: a simple relationship between effective radical concentration and measured dosimeter response; a simple, rapid and non-destructive measurement means; and be unreactive to most proteins. Adenine has been shown to be an effective internal standard dosimeter for HRPF experiments when combined with UV absorbance detection. Upon oxidation, adenine loses UV absorbance, and this loss of UV absorbance is linear with effective hydroxyl radical concentration, as altered by changes in generated OH radical or by variance of radical scavengers. The measured absorbance of adenine is also linear with protein and peptide oxidation products across a wide variety of amino acids, and adenine is unreactive under most conditions [22].
The Fox Radical Dosimeter measures changes in UV 265 nm photometric absorbance during the labeling process. When flash photolysis proceeds, OH radicals are produced and subsequently react with buffer components, including adenine. The oxidation of adenine results in a loss of photometric absorbance and that loss is proportional to effective OH radical load. As illustrated in Figure 4, the Fox Radical Dosimeter provides a convenient real-time account of adenine OH radical attack. 1 mM adenine was subjected to Fox Photolysis using the noted plasma voltages. As shown, the change in adenine absorbance is proportional to applied plasma voltage.
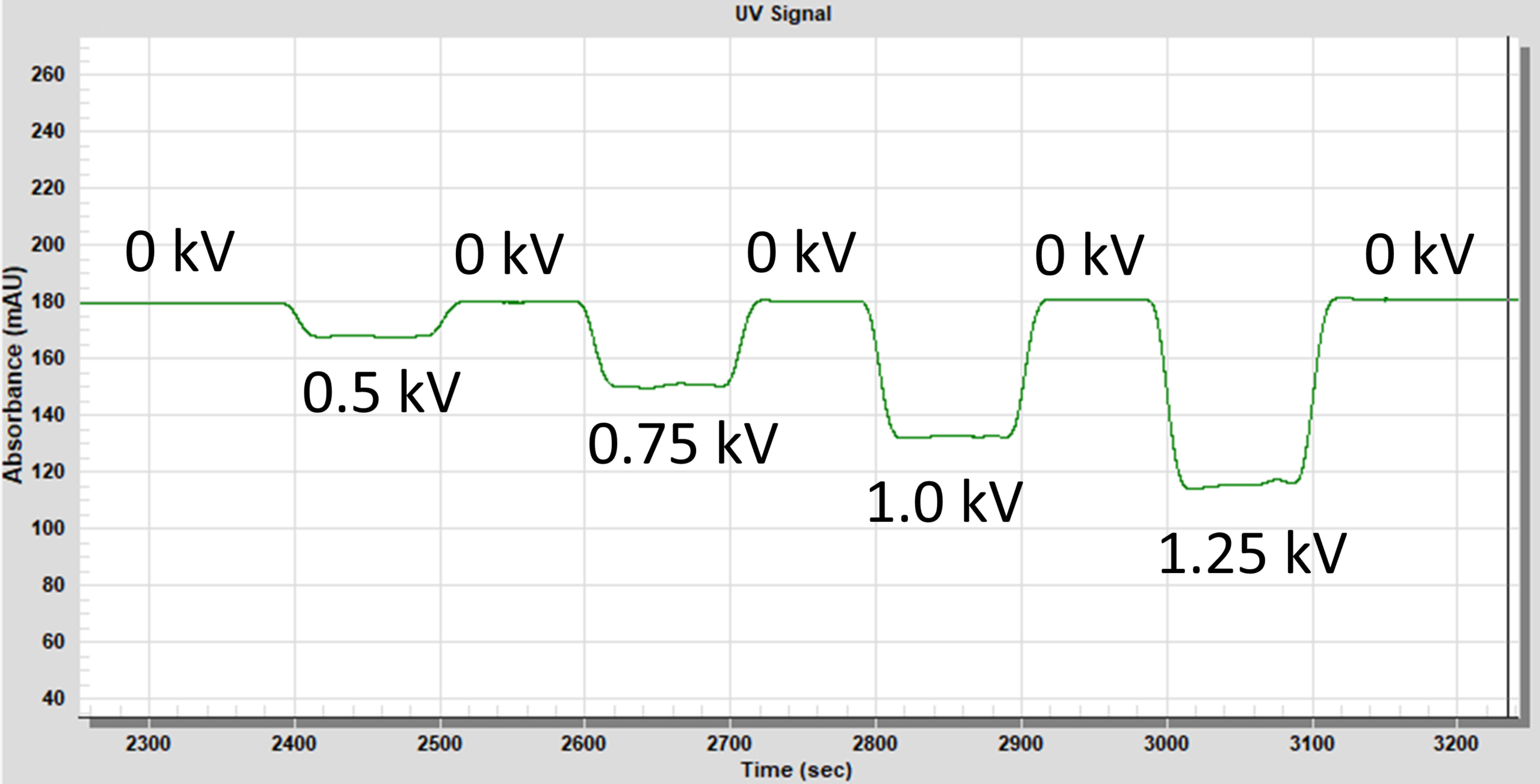
Fig. 4: Real-time trace of adenine UV absorbance change.

Contact us about integrating the Fox Photolysis System in your workflow or sign-up for a pilot project.
FoxWare® HRPF Data Processing Software
Easily perform comparative HOS analysis
As there is no commercial product exclusively tailored for FPOP HRPF HOS data analysis, present day data processing of HRPF results is agonizingly laborious. While broad in scope, today’s proteomics data analysis products fail to adequately inform and fully address specific workflow, chemical labeling/artifacts, and comparative study requirements of HRPF HOS analysis. Researchers are compelled to use a piece-meal approach, arduously stitching together results from multiple packages and manually manipulating data in home brew spreadsheets.
In contrast to broad-based, multi-purpose platforms that miss the mark, FoxWare HRPF Data Processing Software specifically and aptly addresses data processing demands of HRPF HOS analysis. FoxWare Data Processing Software is completely compatible with our either Fox Flash Photolysis or classical laser based FPOP HRPF studies. Intuitive algorithms enabling qualitative and quantitative comparative studies of HOS footprint inform and address key requirements in biopharmaceutical and biosimilar research.
Contact us to learn more about our easy-to-use and integrated FoxWare HRPF Data Processing Software.
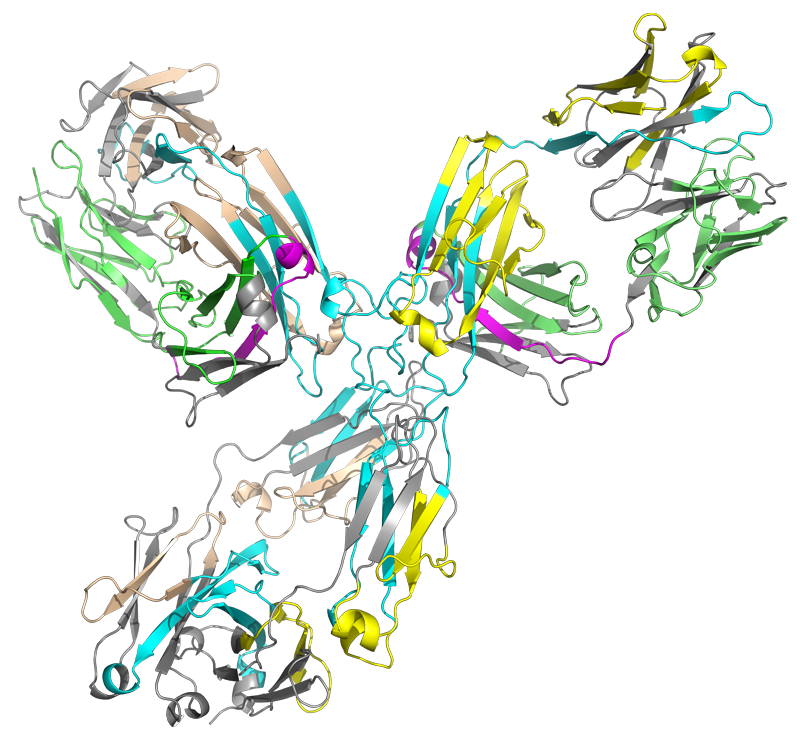
HRPF of conformational changes of a monoclonal antibody biosimilar in sodium phosphate buffer after heat shock at 55°C. Results indicate destabilization of a beta-strand in the light chain along with a probable increase in aggregation mostly mediated by the heavy chain. Grey: No oxidation coverage; Cyan: <2x reduction in oxidation vs. room temp; Magenta: <2x increase in oxidation vs. room temp. No significant change in oxidation is represented by green (light chain) or yellow (heavy chain).

Contact us to learn more about the FOX Automated Product Collector.
Fox® Automated Product Collector
Automatically collect and deposit labeled samples
The Fox Product Collector is an easy-to-use compact device that automatically collects and deposits labeled sample into designated microtubes, eliminating sample collection work load while ensuring maximum product purity. Product Collector activity is controlled by an easy-to-use software program that also coordinates the functionalities of the Photolysis System, Radical Dosimeter, and integrated microfluidic system, obviating the need to attentively determine photolysis processing time to insure proper collection of labeled product without undue introduction of unlabeled sample. User selected product volume is precisely delivered to collection tubes containing quenching solution, enabling reproducible and predictable quenching for each labeled sample.