Spotlight on Protein Aggregation
Understanding protein-protein interactions to develop high-concentration biotherapeutics
Facing the Noncompliance Challenge
Therapeutics which require protracted and often painful intravenous administration can result in medication noncompliance by patients. When the drug delivery experience is unpleasant, avoidance is common, especially among patients with chronic disease.
In response to this challenge, biopharmaceutical companies strive to develop antibodies and other protein formulations containing high concentrations of therapeutics so that a single, small intramuscular or subcutaneous injection is efficacious.
However, high antibody concentration pushes the molecules closer together causing molecular crowding. In turn, crowding increases the propensity for protein-protein interactions that can disrupt the hydrogen bonding matrix, induce protein misfolding, and can eventually manifest as antibody aggregation.
When developing high concentrations of antibodies, it is essential to pinpoint the interfacial domains of protein interaction by conducting protein aggregation studies. By understanding antibody Higher Order Structure (HOS), the antibody formulation can be optimized to limit aggregation.
Identifying Interfacial Domains of mAb Aggregation Using Fox-Based HRPF
The Flash Oxidation (Fox®) Protein Footprinting System is gaining momentum in protein interaction studies because of its robust, yet relatively simple workflow that produces actionable results such as identifying excipients that can stabilize high concentrations of antibodies by limiting protein intermolecular interactions.
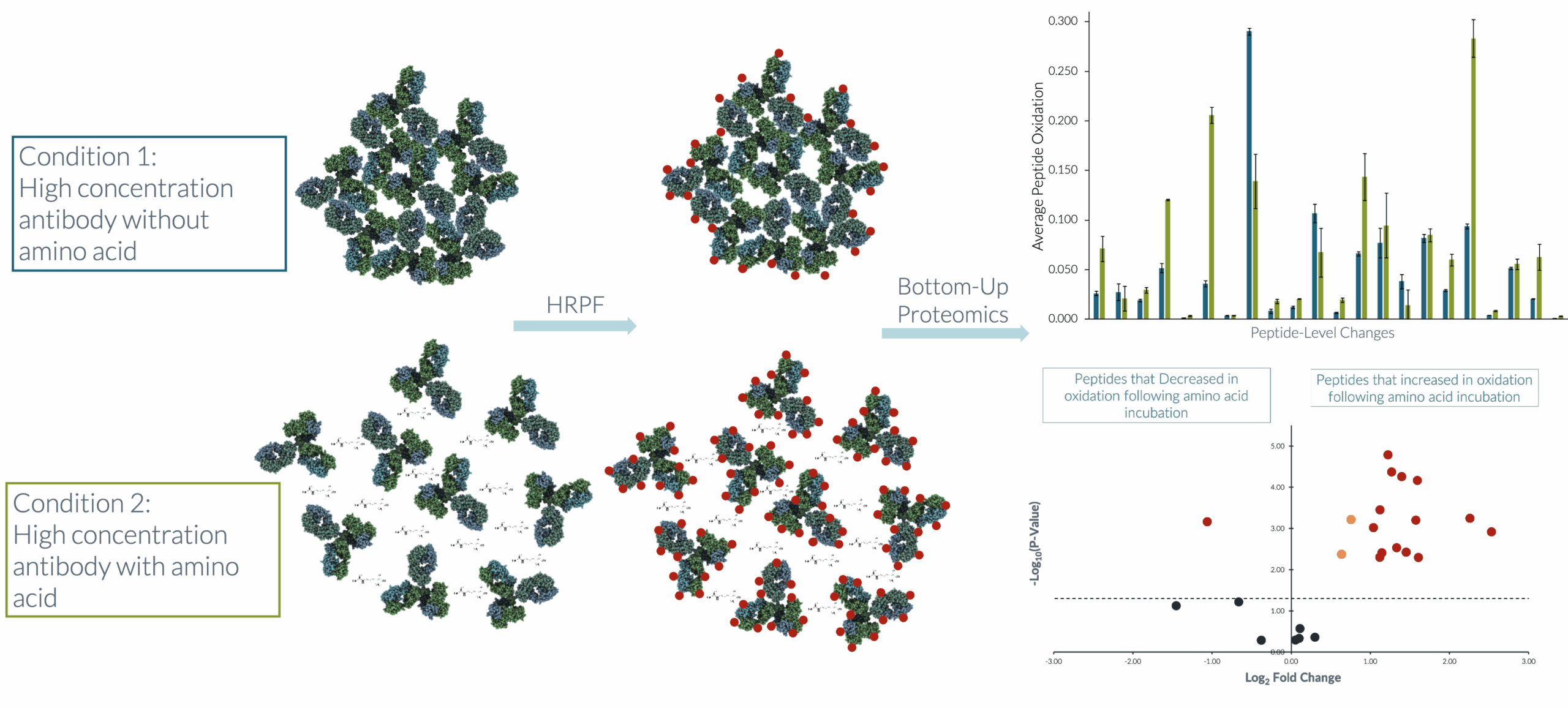
A differential Fox-based HRPF experiment compared the change in oxidation between an aggregated antibody formulation and a non-aggregated formulation that contained an amino acid excipient. The results showed that peptides involved in the intermolecular interactions for the aggregated samples were protected from •OH modification, whereas with the addition of the amino acid, many of those same peptides were exposed to •OH attack.
Employing a volcano plot, the peptides with a significant change in oxidation were easily identified. Several peptides significantly increased in oxidation following amino acid addition. The figure shown highlights the substantial intermolecular interactions that predominated and were ultimately disrupted by the addition of the amino acid. Armed with this powerful data, drug researchers can make further improvements to antibody design and formulation.
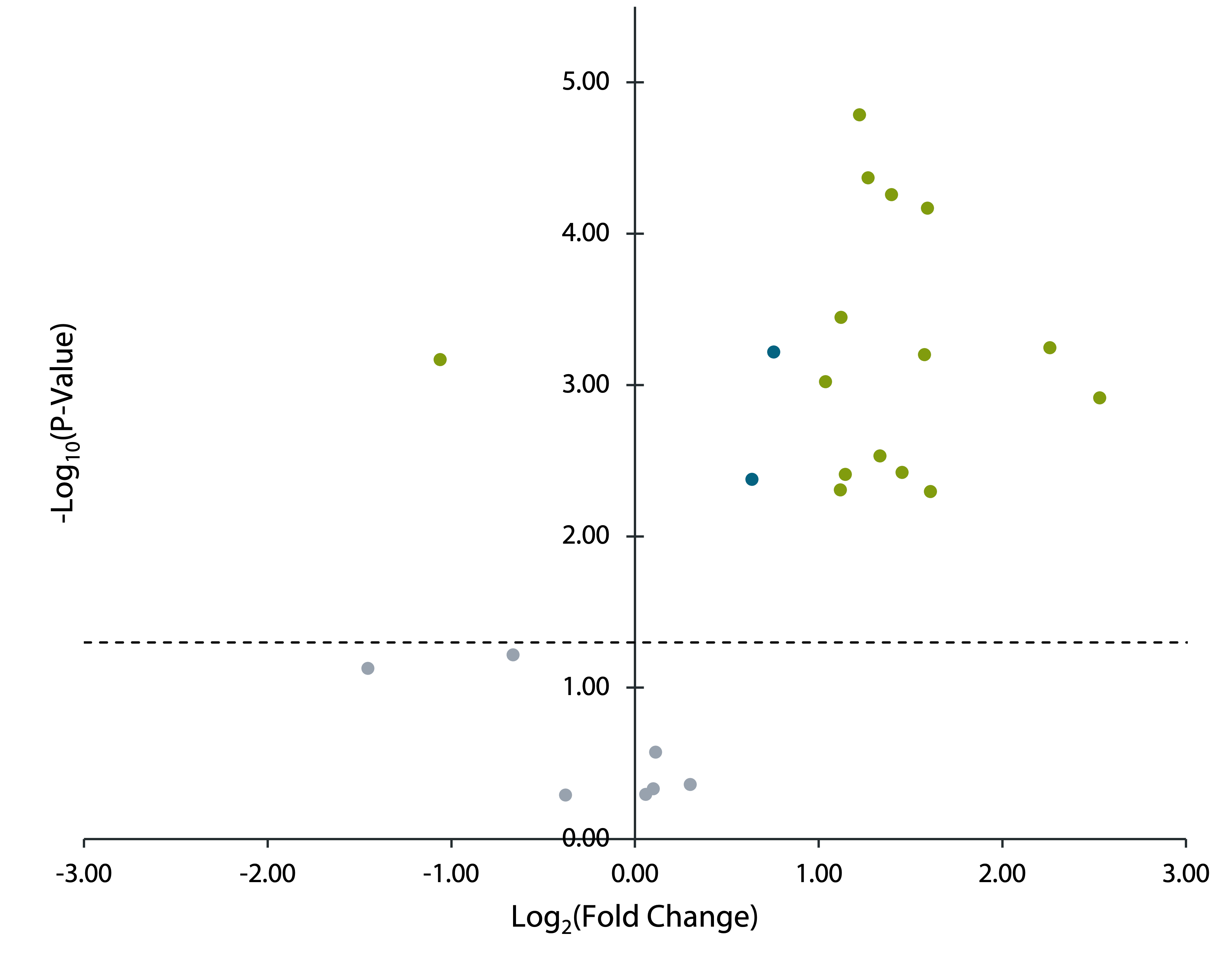
Volcano plot comparing the change in oxidation before and after the addition of an amino acid.
Peptides with a p-value of ≤0.05 are plotted above the dotted line. Peptides highlighted in blue have a fold change of less than two and peptides highlighted in green have a fold change equal to or greater than two. Peptides with a positive fold change increased in oxidation following the amino acid addition while a negative fold change indicates a decrease in oxidation.
Amino Acids Stabilize Proteins Minimizing Intermolecular Interactions
Researchers study antibody aggregation using hydroxyl radical protein footprinting (HRPF) to identify surface domains involved in intermolecular interactions that drive aggregation. This insight enables the rational design of more stable biologics and the strategic use of excipients to minimize aggregation, improving safety, efficacy, and shelf life of therapeutic antibodies.